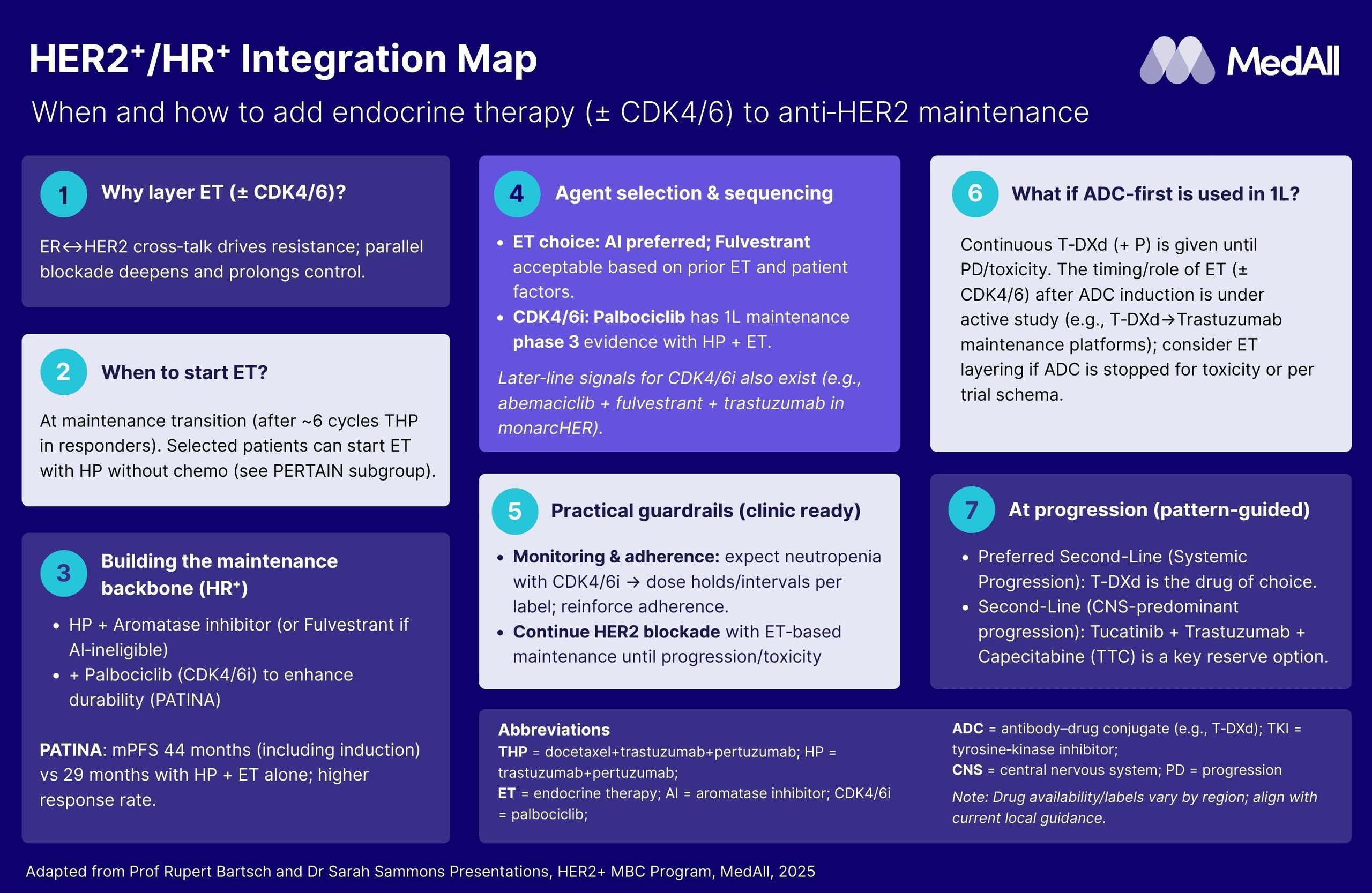
1L HER2+ / HR+ MBC: Integration Map
After participating you can claim your CME credit here: https://forms.gle/Hjgg2iyd6v1PTfeh9
Accreditation: 0.25 AMA PRA Category 1 Credits™
This program is supported by an independent education grant from Pfizer Global Medical Grants. This online education program has been designed solely for healthcare professionals in the USA. The content is not available for healthcare professionals in any other country.
Learning objectives
**
- Explain evolution and rationale for maintenance de-escalation in 1L HER2⁺ MBC:**
- Summarise the shift from CLEOPATRA’s model to current de-escalation trials, describing survival and QoL gains.
- Define optimal timing to begin maintenance therapy
- Identify clinical markers for safely shortening or re-escalating cytotoxic therapy in practice.
2. Understand the role of endocrine therapy ± CDK4/6 inhibitors in HE2R+/HR+ MBC 1L setting:
- Review ER–HER2 cross-talk and key data (including PERTAIN, PATINA, monarcHER) supporting endocrine layering.
- Select and sequence ET agents and CDK4/6 inhibitors based on patient factors, exposure, and toxicity.
- Manage resistance and adherence through dual-targeting strategies and patient engagement.
3. Evaluate multi-drug maintenance strategies including TKIs and ADCs:
- Evaluate the latest evidence for adding TKIs or switching/layering ADCs.
- Balance efficacy endpoints, CNS benefits, and toxicity profiles when choosing TKIs vs ADCs.
- Sequence and dose-modify multi-drug regimens.
4. Apply patient-centred, QoL-focused decision-making to personalize maintenance regimens:
- Match regimen intensity to tumour biology, disease tempo and CNS risk.
- Adjust doses, schedules, or drug combinations to mitigate toxicity and align with patient goals.
- Use shared-decision tools to accommodate comorbidities, lifestyle, and survivorship priorities.
5. Evaluate guidelines and latest evidence to understand the treatment paradigm and 1-L standard of care evolution for patients with HER2+ MBC
- Synthesize current guideline recommendations and ongoing studies.
- Map decision points from induction through maintenance and later-line transitions.
Continuing Education Information
Commercial support: This activity received monetary support through an independent education grant from Pfizer Global Medical Grants. This online education program has been designed solely for healthcare professionals in the USA. The content is not available for healthcare professionals in any other country.
This continuing education activity will be provided by AffinityCE and MedAll. This activity will provide continuing education credit for physicians. A statement of participation is available to other attendees.
Disclosures
Below is a listing of all individuals who are involved in the planning and implementation of this accredited continuing education activity.
Dr Rupert Bartsch has disclosed financial relationships with the following ineligible companies within the past 24 months: Amgen, AstraZeneca, Bristol Myers Squibb (BMS), Daiichi Sankyo, Eisai, Eli Lilly, Grünenthal, MSD, Novartis, Roche Pharmaceuticals, and Gilead Sciences. These relationships include advisory roles, lecture honoraria, research support, and travel support.
Dr Sarah Sammons has disclosed financial relationships within the past 24 months with the following ineligible companies: Daiichi Sankyo, Relay Therapeutics, Seagen, Sermonix, AstraZeneca, Gilead, Eli Lilly, Incyclix, Pfizer, and Novartis. These relationships include research funding and consulting/advisory roles.
These disclosures are made in accordance with ACCME standards to ensure transparency and objectivity in continuing education.
AffinityCE/MedAll staff and the planners and reviewers of this educational activity have no relevant financial or non-financial interests to disclose.
Mitigation of Relevant Financial Relationships
AffinityCE adheres to the ACCME’s Standards for Integrity and Independence in Accredited Continuing Education. Any individuals in a position to control the content of a CME activity, including faculty, planners, reviewers, or others, are required to disclose all relevant financial relationships with ineligible companies. Relevant financial relationships were mitigated by the peer review of content by non-conflicted reviewers prior to the commencement of the program.
Activity Accreditation for Health Professions
Physicians
This activity has been planned and implemented in accordance with the accreditation requirements and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of AffinityCE and MedAll. AffinityCE is accredited by the ACCME to provide continuing medical education for physicians.
AffinityCE will designate this enduring material for a maximum of 0.25 AMA PRA Category 1 Credits™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Other Professionals
All other health care professionals completing this continuing education activity will be issued a statement of participation indicating the number of hours of continuing education credit. This may be used for professional education CE credit. Please consult your accrediting organization or licensing board for their acceptance of this CE activity.
System Requirements
Mobile device (e.g., large-format smart phone; laptop or tablet computer) or desktop computer with a video display of at least 1024 × 768 pixels at 24-bit color depth, capable of connecting to the Internet at broadband or faster speeds, with a current version Internet browser and popular document viewing software (e.g., Microsoft Office, PDF viewer, image viewer) installed. Support for streaming or downloadable audio-visual materials (e.g., streaming MP4, MP3 audio) in hardware and software may be required to view, review, or participate in portions of the program.
Unapproved and/or off-label use disclosure
AffinityCE/MedAll requires CE faculty to disclose to the participants:
- When products or procedures being discussed are off-label, unlabeled, experimental, and/or investigational (not US Food and Drug Administration [FDA] approved); and
- Any limitations on the information presented, such as data that are preliminary or that represent ongoing research, interim analyses, and/or unsupported opinion.
CME Inquiries
For all CME policy-related inquiries, please contact us at ce@affinityced.com.
Participation Costs
There is no cost to participate in this program.
This continuing education activity is active starting September 29th 2025 and will expire on February 1st 2027. Estimated time to complete this activity: 15 minutes.
Continuing Education Information
Commercial support: This activity received monetary support through an independent education grant from Pfizer Global Medical Grants. This online education program has been designed solely for healthcare professionals in the USA. The content is not available for healthcare professionals in any other country.
This continuing education activity will be provided by AffinityCE and MedAll. This activity will provide continuing education credit for physicians. A statement of participation is available to other attendees.
Disclosures
Below is a listing of all individuals who are involved in the planning and implementation of this accredited continuing education activity.
Dr Rupert Bartsch has disclosed financial relationships with the following ineligible companies within the past 24 months: Amgen, AstraZeneca, Bristol Myers Squibb (BMS), Daiichi Sankyo, Eisai, Eli Lilly, Grünenthal, MSD, Novartis, Roche Pharmaceuticals, and Gilead Sciences. These relationships include advisory roles, lecture honoraria, research support, and travel support.
Dr Sarah Sammons has disclosed financial relationships within the past 24 months with the following ineligible companies: Daiichi Sankyo, Relay Therapeutics, Seagen, Sermonix, AstraZeneca, Gilead, Eli Lilly, Incyclix, Pfizer, and Novartis. These relationships include research funding and consulting/advisory roles.
These disclosures are made in accordance with ACCME standards to ensure transparency and objectivity in continuing education.
AffinityCE/MedAll staff and the planners and reviewers of this educational activity have no relevant financial or non-financial interests to disclose.
Mitigation of Relevant Financial Relationships
AffinityCE adheres to the ACCME’s Standards for Integrity and Independence in Accredited Continuing Education. Any individuals in a position to control the content of a CME activity, including faculty, planners, reviewers, or others, are required to disclose all relevant financial relationships with ineligible companies. Relevant financial relationships were mitigated by the peer review of content by non-conflicted reviewers prior to the commencement of the program.
Activity Accreditation for Health Professions
Physicians
This activity has been planned and implemented in accordance with the accreditation requirements and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of AffinityCE and MedAll. AffinityCE is accredited by the ACCME to provide continuing medical education for physicians.
AffinityCE will designate this enduring material or a maximum of 0.25 AMA PRA Category 1 Credits™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Other Professionals
All other health care professionals completing this continuing education activity will be issued a statement of participation indicating the number of hours of continuing education credit. This may be used for professional education CE credit. Please consult your accrediting organization or licensing board for their acceptance of this CE activity.
System Requirements
Mobile device (e.g., large-format smart phone; laptop or tablet computer) or desktop computer with a video display of at least 1024 × 768 pixels at 24-bit color depth, capable of connecting to the Internet at broadband or faster speeds, with a current version Internet browser and popular document viewing software (e.g., Microsoft Office, PDF viewer, image viewer) installed. Support for streaming or downloadable audio-visual materials (e.g., streaming MP4, MP3 audio) in hardware and software may be required to view, review, or participate in portions of the program.
Unapproved and/or off-label use disclosure
AffinityCE/MedAll requires CE faculty to disclose to the participants:
- When products or procedures being discussed are off-label, unlabeled, experimental, and/or investigational (not US Food and Drug Administration [FDA] approved); and
- Any limitations on the information presented, such as data that are preliminary or that represent ongoing research, interim analyses, and/or unsupported opinion.
CME Inquiries
For all CME policy-related inquiries, please contact us at ce@affinityced.com.
Participation Costs
There is no cost to participate in this program.
This continuing education activity is active starting September 29th 2025 and will expire on February 1st 2027. Estimated time to complete this activity: 15 minutes.